
Our certified Software as a Medical Device enables us to improve patient care, simplify clinical workflows and collect new data for researchers.
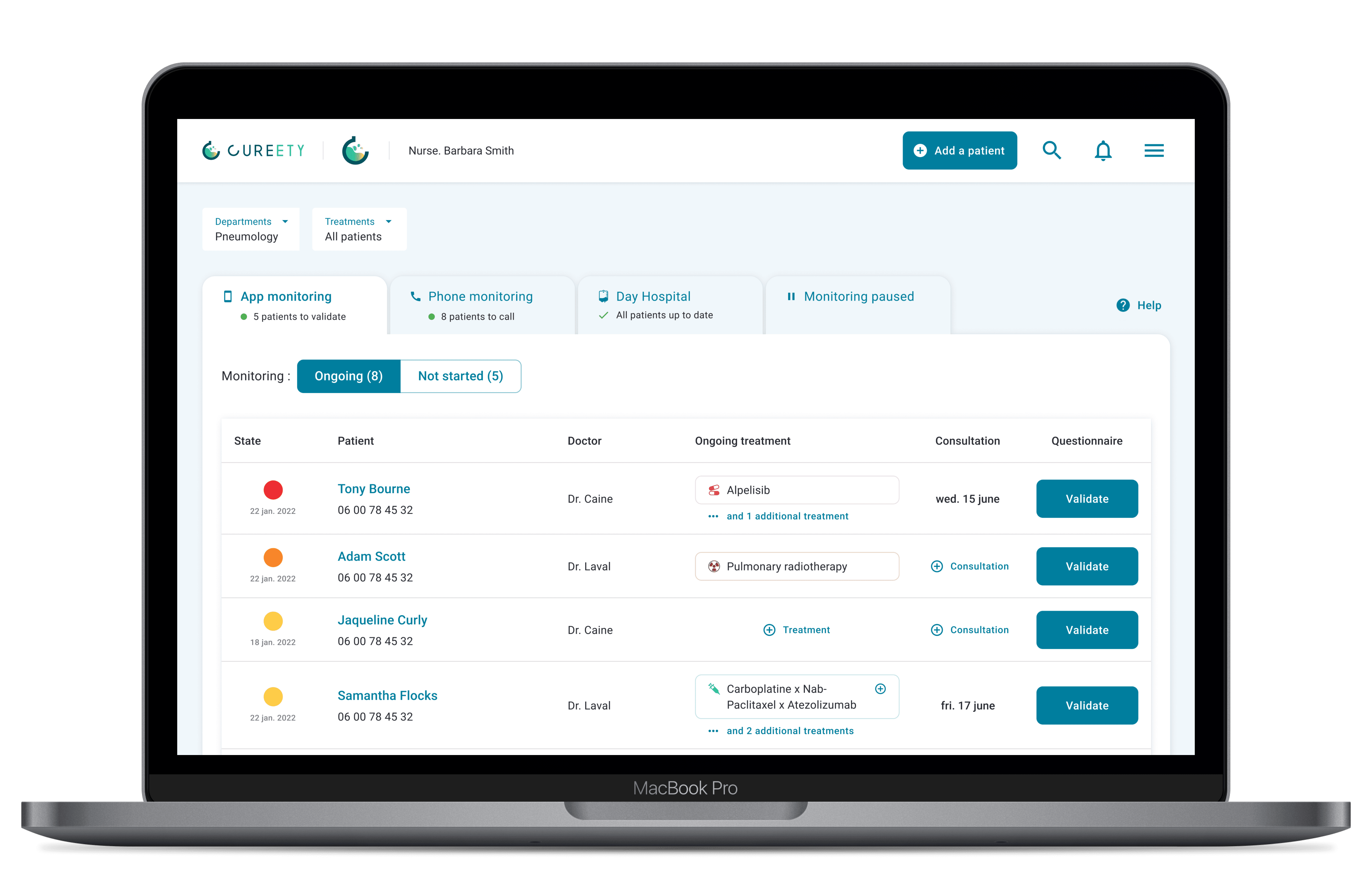
Patients or their clinical team can use the platform independently, on their computer, tablet or cell phone. At regular intervals, patients complete a monitoring questionnaire specific to their treatment or protocol.
After analysis of the responses by our medical device, an alert is generated for the care teams, and an appropriate guidance message is sent to the patient, along with therapeutic advice to help manage grade 1 and 2 adverse events.
Patient information content and digital supportive care are also accessible via the app.
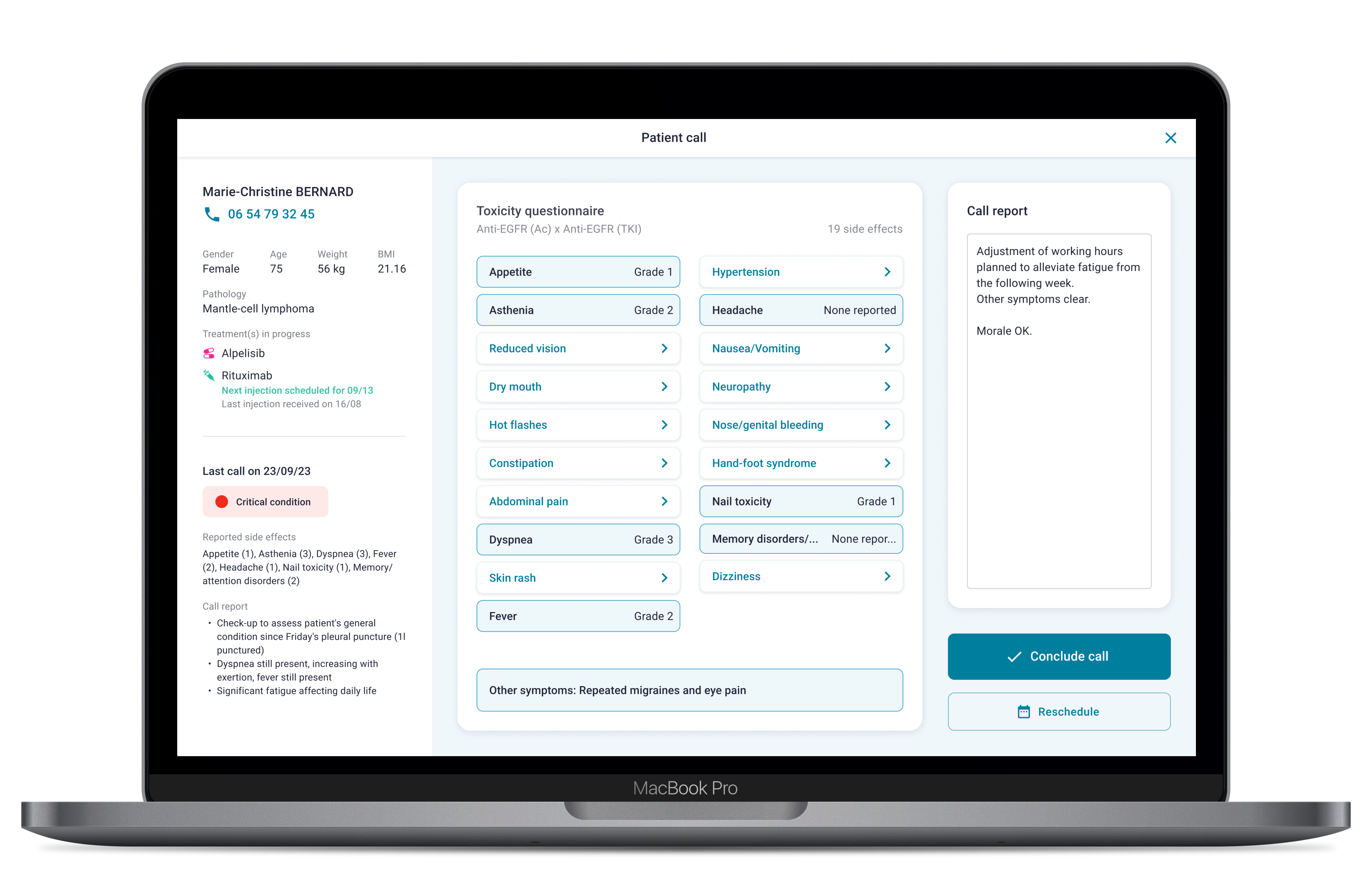
For the few patients who cannot or do not wish to use the application, a phone monitoring module is available.
A member of the care team can enter the patients responses to the questionnaire during a phone call with the patient.
Following analysis of the responses by our medical device, an alert is generated for the care teams. The entire patient cohort can thus be remotely monitored via our platform.
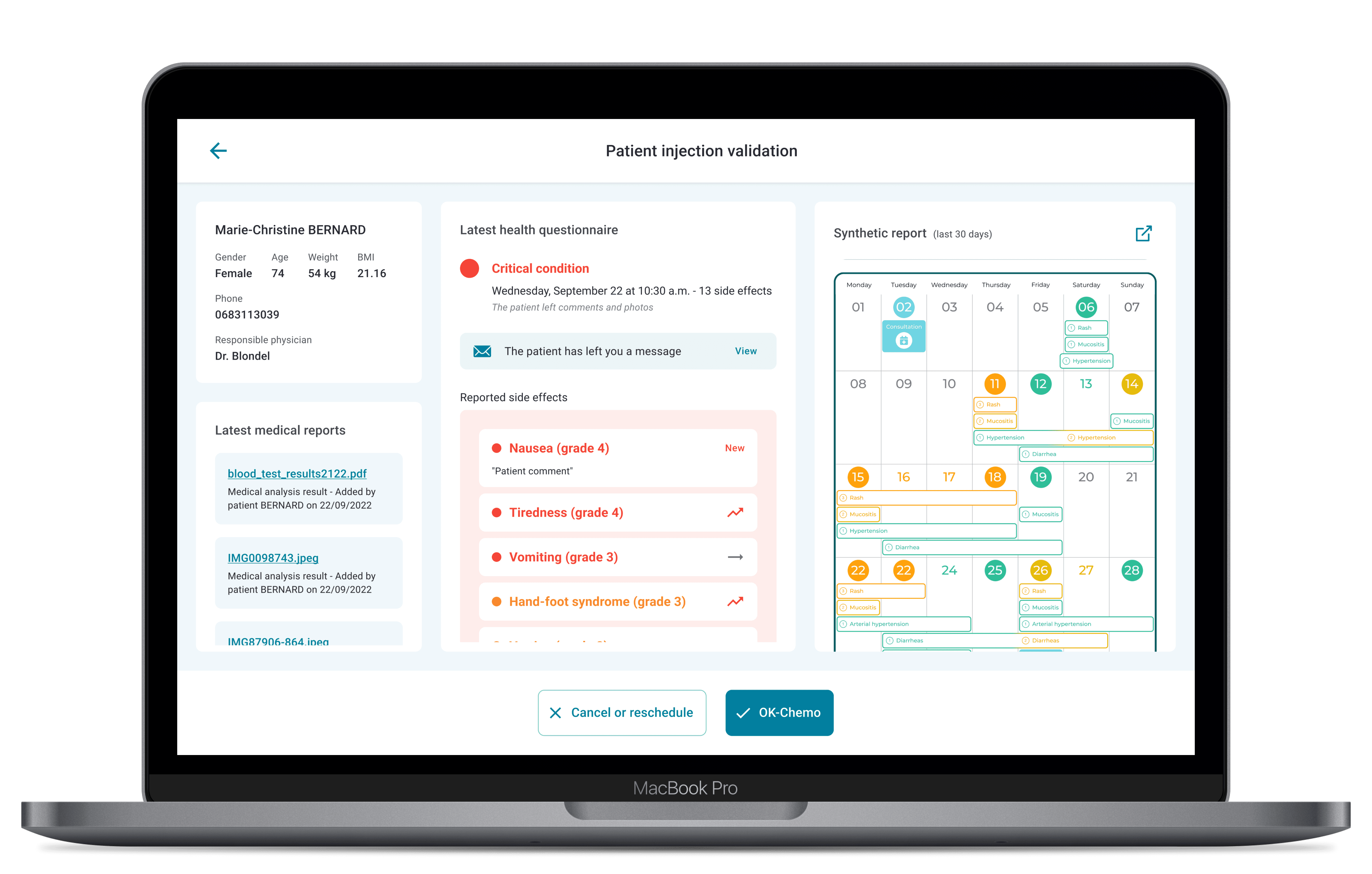
The “OK-Chemo” module collates patients’ reported toxicities and blood test results one or two days before their scheduled appointments.
This is used to pre-validate patients’ chemotherapy appointments at the outpatient department.
Precise symptom monitoring and proactive management
Scheduling a visit in day hospital
Consultation support
Cureety TechCare is a CE certified Medical Device*.
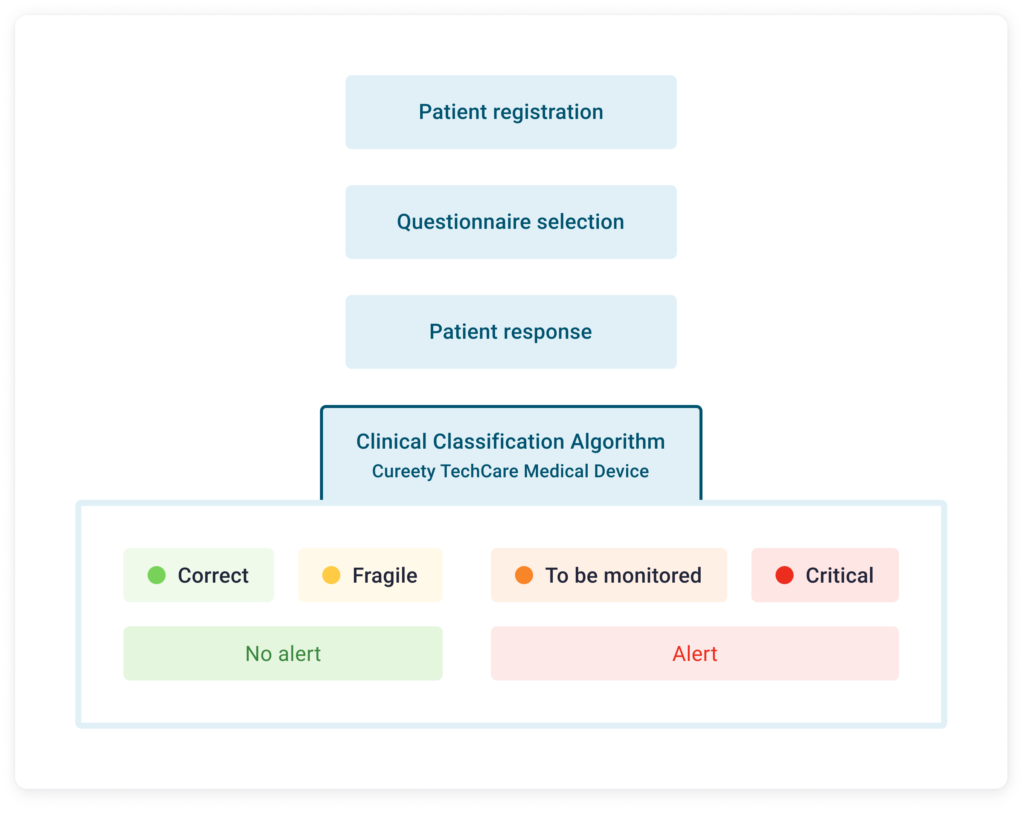
Patient registration
Questionnaire selection
Patient response
Clinical Classification Algorithm
Cureety TechCare Medical Device
No alert
Alert
Cureety offers questionnaires by therapeutic class for systemic treatments and by location for radiation therapy.
The questions and answers are based on the CTCAE standard - Common Terminology Criteria for Adverse Events (objective grading criteria). Each patient response is assigned a number of points. At the end of the questionnaire, the Cureety TechCare algorithm determines the sum of the points associated with each answer, which corresponds to a clinical classification indicative of the patient's state of health.
For patients classified as orange or red, the patient is invited to contact the hospital and an alert is sent to the care team.
CThis class-based approach makes it possible to ask the patient only the relevant questions and to allocate each undesirable event a number of points according to its severity for the therapeutic class in question.
To validate the performance of our algorithm, we have compared the classifications given by expert doctors with the results of the algorithm.
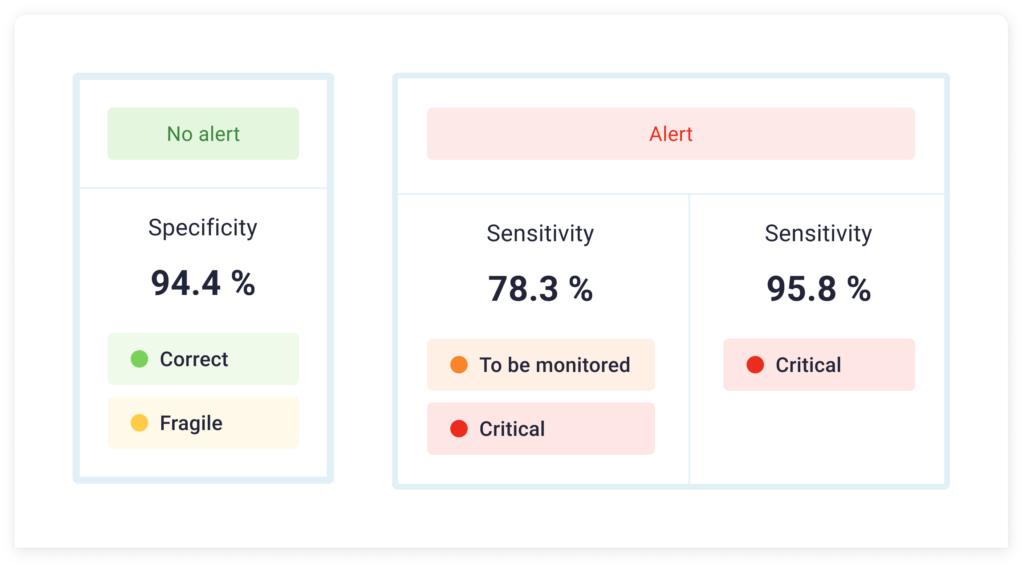
This approach revealed a specificity of 94.4% and a sensitivity of 95.8% for "critical" classifications, and 78.3% for "to be monitored" and "critical" classifications.
This means that our medical device can identify situations requiring medical intervention, generating very few false alarms.
No alert
Specificity
Alert
Sensitivity
Sensitivity
Detecting symptoms before consultations
Improved quality of life
Reduced risk of hospitalization
Improved life expectancy































"The app allows us to maintain a very human relationship with our patients and to be able to respond quickly to their needs."
Dr. Noémie STUDDER, support care and patient pathways manager
“The Cureety platform allows me to manage my patients much more easily and quickly.“
Joana ROSE, coordinating nurse
"We use Cureety as a remote monitoring tool for all patients in the Porte de Saint-Cloud clinical center, for all types of patients, all types of location and all types of treatments, from chemotherapy to immunotherapy, targeted therapy or radiotherapy."
Dr. Thomas FEUTREN, radiation oncologist
Do Hospital Staff Benefit from Remote Monitoring of Patients Receiving Oral/Intravenous Cancer Treatments? Results from the Minerva Study
Alexandre, Le Bail, Champoiseau, Parnot, Pierache, Stanbury, Méric
Real-world Deployment of Patient Monitoring in Oncology Care Centers in France: a Full Retrospective Analysis (ARGOS)
Parnot, Stanbury, Bégin, Champoiseau
A study to assess the diagnostic performance of a digital remote monitoring tool for cancer patients: the POSITEA-VA study.
Parnot, Montestruc. Journal of Clinical Oncology 2023 Jun 1;41(16_suppl):1605. doi:10.1200/JCO.2023.41.16_suppl.1605
Early patient-reported outcomes are a promising predictive factor of cancer progress and outcome in older patients: The EPROFECY study.
Helissey, Riviere, et al. Journal of Clinical Oncology 2022 June 1;40(16_suppl):12045. doi:10.1200/JCO.2022.40.16_suppl.12045
Electronic patient-reported outcomes are a promising predictive factor of prostate cancer patient survival: The Protecty study
Helissey, Riviere, et al. Journal of Clinical Oncology 2022 Feb 20;40(6_suppl):76. doi:10.1200/JCO.2022.40.6_suppl.076
Effectiveness of a digital telemonitoring platform for elderly cancer patient (EP) care
Helissey, Duverger, et al. Annals Oncol 2021;32(S5):S1187. doi: 10.1016/j.annonc.2021.08.1675
Improving cancer patient care with a digital telemonitoring platform: The ConnectPatientToDoctor study
Helissey, Duverger, et al. Journal of Clinical Oncology 2021;39:15_suppl:1581. doi: 10.1200/JCO.2021.39.15_suppl.1581
Effectiveness of electronic patient reporting outcomes, by a digital telemonitoring platform, for prostate cancer care: the Protecty study
Helissey, Parnot, et al.
Front. Digit. Health 2023 May 8;5:1104700. doi:10.3389/fdgth.2023.1104700
Effectiveness of a digital telemonitoring platform for cancer care of older patients: The ConnectElderlyPatientToDoctor study
Rivière, Brureau,et al. Int J Cancer. 2023 Feb 1;152(3):504-510. doi:10.1002/ijc.34196
The Use of Telemedicine in Cancer Clinical Trials: Connect-Patient-to-Doctor Prospective Study
Meghiref, Parnot, et al. JMIR Cancer 2022 Jan 27;8(1):e31255. doi:10.2196/31255